INTRODUCTION
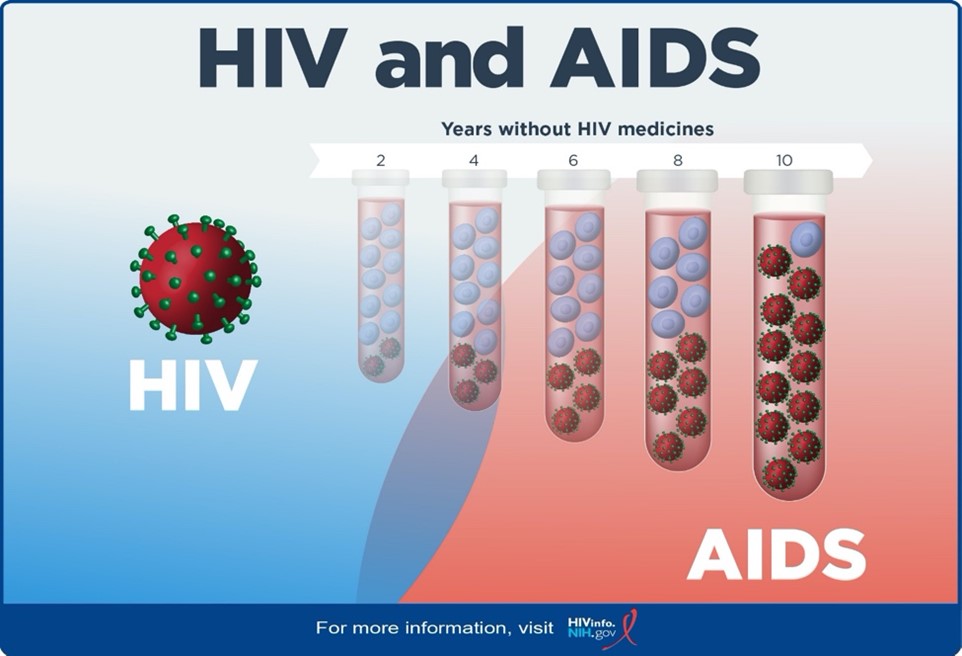
Human Immunodeficiency Virus (HIV) is a retrovirus that attacks the body’s immune system by targeting a specific type of white blood cell, called CD4 cells. CD4 cells help the body fight infection by signaling an immune response when a foreign substance is present. The normal CD4 count range is 500-1500cells/mm3 of blood.
Acquired Immunodeficiency Syndrome (AIDS) is the most advanced stage of the HIV virus. AIDS occur when the CD4 count falls below 200 cells/mm3. Depletion of the CD4 cells will leave the person susceptible to opportunistic infections. Opportunistic infections (OIs) are infections that occur more frequently and are more severe in people with weakened immune systems that do not usually cause disease in normal immunity. Infections are likely to develop as the immune system is compromised. People living with HIV are living longer due to the widespread development of antiretroviral therapy (ART). ART suppresses viral load and improves the function of the immune system. With correct ART therapy, the immune system can function properly and reduce disease progression.
 Reference: HIV and AIDS: The Basics | NIH
Reference: HIV and AIDS: The Basics | NIH
EPIDEMIOLOGY
HIV/AIDS (human immunodeficiency virus infection and acquired immunodeficiency syndrome) was first documented 1981 and has been an ongoing worldwide epidemic. According to the World Health Organization (WHO), there are approximately 38.4 million people living with HIV worldwide, of those, an estimated 1.2 million people 13 years and older are living with HIV in the United States. Although, there have been advances in treatment, allowing those affected to live longer and healthier lives, HIV still disproportionately affects certain populations such as, Blacks/African Americans, sex workers and their clients, men who have sex with men (MSM), injection drug users, and transgender people and their sexual partners. Adults 45-54 and 55 years of age and older accounting for the largest number of persons living with diagnosed or undiagnosed HIV respectively. These age groups also had the highest HIV prevalence rate.12
Among the four regions in the United States, the Southern region has the highest amount of people living with HIV (481,815 persons), while the Northeast region has the highest rate of those living with HIV (411.4 cases per every 100,000 people).
According to Centers for Disease Control and Prevention, 1 in 158 adults in Florida are known to be living with HIV (110,034 people total). Florida has the third-highest number of people living with HIV in the United States following Washington DC at #1 and Georgia at
| Drug Class | Mechanism of Action | Drugs in Class | Common Side Effects | Additional Notes |
| CCR5 Antagonists | Bind to CCR5 co-receptors on CD4 cells and prevent HIV from entering the cell (Stage 1) | Selzentry® (Maraviroc, MVC) | Rash, fever, GI issues
BBW-Hepatotoxicity Contraindications-CrCl <30 AND concurrent use with 3A4 inhibitors/inducers |
Tropism testing required
Major CYP3A4 substrate
|
| Post Attachment Inhibitors | First monoclonal antibody for HIV; blocks entry of HIV into host cells without causing immunosuppression (Stage 1)
Inhibits HIV from attaching to host immune system CD4 cells by binding directly to the glycoprotein 120 (gp120) |
Trogarzo® (Ibalizumab-uiyk, IBA)
Rukobia® (fostemsavir, FTR) |
Used for multi-drug resistance with additional agents Trogarzo™ has no major drug-drug interactions and have no cross-resistance with other ARTs
Loading dose 2000 mg IV Maintenance dose 800 mg IV every 2 weeks If patient misses maintenance dose by >3 days, must repeat loading dose
Rukobia is taken orally – 600mg PO BID |
|
| Fusion Inhibitors | Blocks the fusion of HIV into CD4 cell membrane by blocking conformational change in GP41 required for membrane fusion & entry into CD4 (Stage 2) | Fuzeon® (Enfurvirtide, T20) | Side effects – diarrhea, nausea, fatigue | Dose: 90mg SubQ BID
Rotate Injection Sites |
| Nucleoside Reverse Transcriptase Inhibitors (NRTIs)
|
Competitively binds to the HIV enzyme reverse transcriptase and inhibits conversion of HIV RNA to DNA (Stage 3) | Abacavir (ABC) (Ziagen®)
Didanosine (ddI) (Videx®) Emtricitabine (FTC) (Emtriva®) Lamivudine (3TC) (Epivir®) Stavudine (d4T) (Zerit®) withdrawn 2020 Tenofovir (TDF or TAF) (Viread® or Vemlidy®) Zalcitabine (ddC) (Hivid®) withdrawn 2005 Combination NRTI Zidovudine (ZDV, AZT) (Retrovir®) 3TC/ABC (Epzicom®) 3TC/ABC/ZDV (Trizivir®) 3TC/ZDV (Combivir®) 3TC/TDF (Cimduo®, Temixys®) FTC/TDF (Truvada®) FTC/TAF (Descovy®) |
Black Boxed Warnings
Lactic acidosis, Severe hepatomegaly (seen more with Zidovudine, Stavudine, and Didanosine) Decreased Bone Mineral Density and renal function (Seen more in TDF)
|
Backbone of combination therapy
No major CYP interactions If starting patient on Abacavir must test for HLA-B*5701 prior to initiation Low barrier to resistance Do not use emtricitabine and lamivudine together (structures too similar) |
| Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs)
|
Non-Competitively binds to the HIV enzyme reverse transcriptase and
Inhibits conversion of HIV RNA to DNA |
Delavirdine (DLV) (Rescriptor®)
Doravirine (DOR) (Pifeltro®) Efavirenz (EFV) (Sustiva®) Etravirine (ETR) (Intelence®) Nevirapine (NVP) (Viramune®) Rilpivirine (RPV) (Edurant®) |
Adverse Events: hepatotoxicity, rash:Steven’s Johnson Syndrome (SJS), Toxic Epidermal Necrolysis (TEN), Lipoatrophy | No renal dose adjustments needed (Avoid Atripla and Complera if CrCl < 50mL/min)
Primarily CYP3A4 subtrates Low barrier to resistance Food requirements: With food – Etravirine, Rilpivirine Without food – Efavirenz Increased risk for CNS side effects |
| Integrase Strand Transfer Inhibitors (INSTIs)
|
block the integrase enzyme needed for viral DNA to integrate with the host cell DNA/human genome (Stage 4) | Bictegravir
Cabotegravir (CAB) (Vocabria®) Dolutegravir (DTG) (Tivicay®) Elvitegravir (EVG) Raltegravir (RAL) Isentress® |
weight gain, increased creatine phosphokinase, headache, insomnia | Space 2 hours before or 6 hours after cation-containing antacids or laxatives, sucralfate, iron or calcium supplements
|
| Protease Inhibitors (PIs)
|
work by inhibiting HIV protease and rendering the enzyme incapable of cleaving the Gag-Pol polyprotein preventing assembly and maturation of the virus (Stage 7)
|
Amprenavir (APV) (Agenerase®) discontinued 2004
Atazanavir (ATV) (Reyataz®) Atazanavir/cobicistat (ATV/c) (Evotaz®) Darunavir (DRV) (Prezista®) Darunavir/cobicistat (DRV/c) (Prezcobix®) Fosamprenavir (FPV) (Lexiva®) Indinavir (IDV) (Crixivan®) Lopinavir/ritonavir (LPV/r) (Kaletra®) Nelfinavir (NFV) (Viracept®) Ritonavir (RTV) (Norvir®) Saquinavir (SQV) (Invirase®) Tipranavir (TPV) (Aptivus®) |
GI disturbances, metabolic complications (insulin resistance, lipohypertrophy, hyperlipidemia), | High barrier to resistance
All PIs are substrates of CYP3A4 Recommended that all PIs be taken with a pharmacokinetic booster to increase levels of the PI Ritonavir is a protease inhibitor and used only for its potent CYP3A4 inhibition to increase (boost) the level of other PIs. Cobicistat is not a protease inhibitor but a strong CYP3A4 inhibitor. FDA-approved to pharmacokinetically enhance levels of Atazanavir and Darunavir. |
| Capsid Inhibitors
|
Interferes with multiple steps in the HIV life cycle by inhibiting replication by directly binding to capsid protein (p24) | Lenacapavir, LEN (Sunlencaâ) | nausea, injection site reactions | Long-acting subcutaneous injection given every 6 months
Contraindicated with strong CYP 3A4 inducers |
| SINGLE TABLET REGIMENS | |||
| BIC/FTC/TAF (Biktarvy®)
|
DRV/cobi/FTC/TAF (Symtuza®)
|
DTG/3TC/ABC (Triumeq®)
|
DTG/RPV (Juluca®)
|
| DTG/3TC (Dovato®)
|
DOR/3TC/TDF (Delstrigo®)
|
EFV/FTC/TDF (Atripla®)
|
EFV/3TC/TDF (Symfi® or Symfi Lo®)
|
| EVG/cobi/FTC/TAF (Genvoya®)
|
EVG/cobi/FTC/TDF (Stribild®)
|
RPV/FTC/TAF (Odefsey®)
|
RPV/FTC/TDF (Complera®)
|


No Comments