INTRODUCTION
The percentage of Americans taking medications has increased over the years. Seventy percent of individuals in the U.S. take at least one medication per day, and more than half of all Americans take two. There is at least one death in the U.S. daily as a result of a medication error, and approximately 1.3 million people annually are injured due to medication errors. Each year, the Food and Drug Administration (FDA) receives more than 100,000 reports of medication errors. Medication errors occur in many settings, including pharmacies, hospitals, and patient homes. According to the Journal of Community Hospital Internal Medicine Perspectives, more than seven million Americans have been impacted by medication errors on an annual basis.
As a part of the biennial renewal, pharmacists and pharmacy technicians are required to complete a two (2) hour Florida Board of Pharmacy approved course on Medication Errors. The course must contain the following components: (a) root-cause analysis; (b) error reduction and prevention; (c) patient safety. The two-hour program may be applied towards the thirty-hour continuing education requirement for pharmacists and the twenty-hour continuing education requirement for technicians. (64B16-26.103)
Brain Check!
How many Americans are affected by medication errors annually?
- 7,000
- 100,000
- 5 million
- more than 7 million
DEFINITIONS
Medication
Medications are any chemical substance, natural or synthetic, that has a medical or pharmacologic effect on the body or can be used to treat a disease or injury. Medications are available as a prescription or nonprescription. Prescriptions are authorized be a medical practitioner. Nonprescriptions are sold legally without a physician and can be available over-the-counter. Medications are available in a variety of formulations: tablets, capsules, gels, patches, implants, inhalants, creams, injections, powders, lotions, creams, ointments, effervescent granules, aerosols, rings, suppositories.[GOK2]
Error
An error is simply something that is wrong or incorrect. It could be a mistake in calculation, judgement, speech, writing, or action. It could also be a failure to complete a planned action as intended, or the use of an incorrect plan of action to achieve a given aim.
Medication Error
Medication Error is defined by the FDA as “any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is in the control of the healthcare professional, patient, or consumer” The FDA definition is based on the National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP) definition of medical error as “any error in the process of prescribing, preparing, dispensing, or administering a medication that may cause or lead to inappropriate medication use or patient harm.”
From these definitions we see that medication errors are not only caused by pharmacists, but also by other people involved in the systems of medication use, including healthcare professionals, consumers, and patients. Medication errors involve prescription drugs, nonprescription medications, vitamins and supplements.
Types of Medication Errors
- Prescribing errors (over and under prescribing)
- Failure to prescribe, administer or dispense a medication
- Receiving a medication too early or too late
- Receiving a medication not authorized
- Improper use of a medication
- Dispensing wrong drug, wrong formulation, or a drug with a wrong label
- Administration errors (wrong dose, wrong route, wrong frequency/duration)
- Monitoring therapy failure, failure to alter therapy, or drug interactions
- Improper dispensing/prescribing rules for a medication
- Manufacturing process issues (contaminants or adulterants, wrong/misleading package)
According to Florida Administrative Code 65G-7.006, “A ‘medication error’ is any of the following actions:
- Administration of a wrong medication
- Administration of a wrong dose
- Administration of medication via the wrong route
- Administration of medication for any symptoms, illness, or reason other than the one for which the medication was prescribed
- Failure to administer medication or assist with self-administration medication, to the wrong client
- Failure to immediately and accurately document administration on the MAR
- Failure to fill newly prescribed medications within 24-hours of receipt of the medication
- Administration or assistance with self-administration of an expired or improperly labeled medication
- Failure to conduct an accurate medication count for controlled medications.”
Adverse Drug Events and Adverse Drug Reactions
An adverse drug event (ADE) can be defined as anyinjury resulting from medical intervention related to a drug. This includes medication errors, adverse drug reactions, allergic reactions, and overdoses. ADEs can happen anywhere such as in hospitals, long-term care settings, and outpatient settings. Many ADEs are preventable, which is good news.
An adverse drug reaction (ADR) is a specific type of ADE that is caused by the drug itself. ADRs can be a harmful or an unpleasant reaction, or an unintended response to a drug. Some medication errors result in ADRs but many do not, that is there are times a medication error can result in an ADE and not an ADR.
Root Cause Analysis
A root cause analysis (RCA) is a logical and systematic process used to determine what happened in a situation, why it happened, and how to prevent future occurrences from happening again. Identifying potential specific root causes allows examination and strengthening of barriers to prevent potential errors from reoccurring.
Video
CAUSES OF MEDICATION ERRORS
Medication errors can occur anywhere, from the provider who prescribes the medication, during the dispensing process, or when the medication is administered. Some causes of medication errors include physical, psychological, environmental reasons, and systemic problems. Physical causes may include incorrect calculations of dosages, misreading a prescription, or mistyping a label. Psychological causes may include fatigue, cognitive loss or memory lapse. Environmental causes may include obtaining the wrong medication for the wrong patient, dispensing the incorrect dosage, in addition to lack of proper lighting, heat/cold can cause distractions that lead to errors. Systemic problems occur when medications are incorrectly labeled, mistaking a sound-alike-look-alike medicationin close proximity to another one, lack of bar code scanning system, and other system problems that can lead to medication errors.
MEDICATION ERRORS COST AND STATISTICS
Medication errors, defined as preventable events that may lead to inappropriate medication use or patient harm, lead to 7,000 deaths each year and cost nearly $21 billion. In addition to the rising healthcare costs, the United States has one of the highest rates of medication errors for adults with two or more chronic conditions in the world, according to the Health Affairs 2018 journal.[GOK3]
There are many contributors to the costs associated with medication errors, but many can be prevented through increased communication, enhanced technology, and improved procedures to minimize confusion of high alert. Reducing healthcare costs and improving patient safety are initiatives all healthcare workers should seek.
The following are medication error statistics by settings:
- Home medication errors occur at rates between 2-33%
- Community setting errors occur at 1.5% of all prescriptions
- Hospital stay errors occur at almost 1 in 5 medication doses
- Dosage errors are the most common type of administration error
- Geriatrics are more likely to be affected by medication error due to polypharmacy
INSTITUTE OF SAFE MEDICINE (ISMP) SURVEY
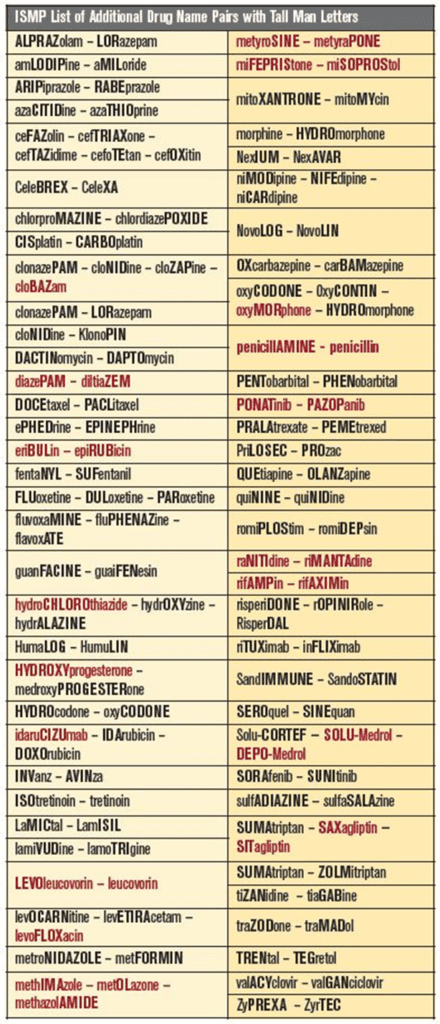
The Institute of Safe Medicine Practice (/ISMP) conducted a study to review the current list of look-alike drug names with tall man (intentionally mixed case) lettering. The ISMP involved healthcare practitioners to identify confusing drug names relevant to their respective practice settings, along with reviewing proposed tall man lettering for possible implementation. The CAPITALIZED and bolded letters should make the drug names distinguishable from one another.
According to the ISMP survey, 95% of respondents felt that the use of tall man letters by the pharmaceutical industry on product and carton labels helped to reduce drug selection errors. The FDA has added three drug name pairs to its list since the last update in 2016.
CISplatin and CARBOplatin
migALAstat and migLUstat
traZODone and traMADol Other possible changes included removing acetoHEXAMIDE, sulfiSOXAZOLE, and TOLBUTamide since these drugs are no longer marketed in the United States. However, FDA did not want to remove these names from its list because the names might still be listed in electronic drug information sources. Also, unless a drug is removed from the market for reasons of safety or efficacy, the drug could still be marketed at a future date.
MEDICATION ERROR CONSEQUENCES
Medication errors can have a significant effect on a patient’s overall health, well-being, and quality of life. Receiving and taking the incorrect medication, or improperly taking the right medication can lead to life threatening side effects, hospitalization, and even death. Adverse drug reactions may present as skin rashes or other skin problems. Some patients may develop new health problems. Each year in the United States, there are 7000-9000 deaths due to medication errors.
Currently there is no consensus if healthcare professionals are liable for medication errors that lead to patient harm. However, healthcare professions should be aware since they may be subject to certain consequences. The professional may have to pay a fine, may lose their job, may lose their license, and depending upon the severity of the case they may have to serve jail time. In many instances it may be difficult to pinpoint who or what caused the medication error as there are many steps involved in the process. *(click for video) Below are some statistics regarding medication errors:
- 7000-9000 Americans die each year from a medication error (NCBI, 2021)
- There is at least one death per day in the U.S. from a medication error. (WHO 2017)
- Medication errors injure more than 1 million people each year in the U.S. (WHO, 2017)
- In hospital settings, thirty percent of injuries are due to prescription drug errors. (Drug Safety, 1986)
Medication Error Prevention
Medication errors can be prevented by carefully considering the systems involved in ordering, prescribing, dispensing, accepting, and administering medications. Many medication errors occur related to some level of human error. While technology can be a valuable tool for reducing human error, it is important to remember that technology is not always a perfect solution. The implementation of technology must be carefully considered to ensure that it is used effectively and does not introduce new risks.
Several ways to prevent medication errors include patient education, increased workplace safety, and improved medication use systems. Patient education and engagement will allow patients to be their own advocates. Patients require basic knowledge to administer, handle and support safe medication use. Patients should clearly understand the importance of why they are taking a medication and the complications that may occur if not in compliance. Patient education allows patients to become more informed and empowers them to be advocates of their own health. Patient engagement tools from the Agency for Healthcare Research and Quality (AHRQ) Health Universal Precautions Toolkit include:
- Communicating clearly
- Using the teach back method
- Following up with patients
- Addressing language barriers
- Considering beliefs, cultures, and customs
- Using education materials
- Encouraging questions
- Helping patients remember how and when to take their medicine
- Obtaining patient feedback
A safe workplace environment not only protects employees but also customers and patients as well. Workplace safety involves adequate space, and a clean, well-lit area; all which may reduce the possibility of medication errors. Automation and bar code fill procedures may also contribute to an orderly work area. The pharmacy area should provide adequate space, proper storage areas, and drug labels clearly outlined on the shelves. A safe workplace is a happy workplace and will create a more comfortable and conducive environment for employees to perform their jobs effectively.
Electronic Medical Records (EMRs) are digital versions of the paper charts in clinician offices, larger clinics, and hospitals. EMRs contain notes and information collected by and for healthcare workers and are for diagnosis, treatment, and patient care. EMRs can be potentially more valuable than paper records by enabling providers to track data over time, identify patients for preventive visits and screenings, monitor patients, and improve health care quality. Electronic medical records can identify potential drug-drug interactions and alert for correct dosages and allergies. Automated dispensing cabinets can be helpful for high-risk medications in healthcare facilities. Purposefully well thought out and carefully implemented computer software and applications can help to minimize medication errors.
Additional medication error prevention strategies include the 5 Rights of Medication Administration
Before dispensing a medication or giving a loved one any medication, remember to do the “5 Rights” – Right Person, Right Medication, Right Dose, Right Time, and Right Route.
1. Right Person: Make sure that the name on the bottle matches who will be receiving the medication. This question may seem simple and self-explanatory but there may be others with a similar name. You may also want to ask for a second form of verification, such as the date of birth, to confirm it is the correct person.
2. Right Medication: Be sure to verify that the medication is correct. Patients may be taking multiple medications and may get confused. There are times the medication may change to a generic from a brand, or a different generic manufacturer for the same generic medication. Keep in mind that manufacturers of the medications may change without notice, thus changing its shape, size, and/or color. Do not assume what a medication is just by the way it looks. Be sure to ask the physician if there is uncertainty for what the medication is for or if a generic medication was prescribed as a substitute. Ask the physician if a prior medication was to be discontinued. If discontinued, be sure to inform the patient to discard the old medication properly. If it is not discarded, it may cause potential problems. Inform the patients to not use expired medication bottles to store new medication. Keep all medications in their respective bottles unless putting it directly from the bottle into their pill box.
3. Right Dose: Verify the correct dose of each medication. Physicians sometimes increase or decrease the dosages of medications, depending upon the patients’ response to it. Be aware of the current dose and do not adjust doses without consulting a physician!
4.Right Time: Inform the patient of the correct time of the medications. Medications prescribed twice a day, three times a day or four times a day should be explained as to when to take them and how many hours apart. For example, three times a day is every 8 hours. Patients should be informed if medications should be taken with or without food and informed of any medications causing drowsiness to avoid driving. A physicians should be consulted for any concerns about how a medication is affecting the patient.
5. Right Route: Always inform the patient about how the medication should be taken (orally, eye drops, ear drops, injectable, etc.) and inform them to follow the instructions properly. There are often mix ups with ear drops and eye drops. Be sure the medication is clearly identified with the auxiliary labels indicating ‘for the ear’ or ‘for the eye’.
The “5 Rights” help healthcare professionals and caregivers remember the importance of carefully and correctly reading medication labels and hopefully minimize any medication errors.
Other strategies to prevent medication errors include
- Repeating verbal orders and spell the name of the drug back to the doctor’s office
- Avoiding abbreviations; Avoid using trailing zeros for example, 25mg versus 25.0mg
- Using brand and generic names to avoid any confusion
- Verifying illegible orders
Brain Check!
Which is not one of the 5 Rights?
a. Right person
b. Right physician
c. Right medication
d. Right route
QUALITY RELATED EVENTS
Medication Quality Related Events (QREs) are any event that has the potential to cause harm to a patient. There are several types of errors that may range from the prescribing physician to the dispensing pharmacy to the caregiver or patient. An error is considered “harm” if it is not intercepted before it reaches the patient. While an intercepted error may not cause a patient harm, it may still be considered a quality related event. Ideally, an intercepted QRE will be investigated as the potential to lead to harm could still occur.
There are several important pieces of information that the pharmacist, or pharmacy technician, should include when reporting a QRE
- Description of the QRE
- Date and time QRE occurred along with the date and time the incident was reported
- Who discovered the QRE (ex. Pharmacist, Pharmacy tech, Patient, )
- Degree of harm and status of the patient (potentially could range between ‘error did not reach the patient’ to ‘death’)
- Notification to the treating physician and response
- Medication involved and retrieval of the drug (ex. Administration, follow-up, etc.)
- Identify other staff or caregivers involved
Ideally, the QRE report is a confidential document and should not be shared with unauthorized personnel. Additional items that could be noted in the QRE document include:
- Type of QRE (wrong patient, wrong drug, incorrect strength or dosage form, duplicate therapy, drug utilization, drug interaction, allergy, etc.)
- Staff on duty and staff member who reported the incident
- Location of the incident
- Action provided
- Level of prescription volume
- Level of telephone call volume
- Frequency of interruptions
- Wait times
- Environmental concerns (lighting, noise, distractions, storage etc.)
- QRE interpretation (transcription error, look alike-sound alike drugs
- Other factors involved (computer system, software, electronic prescriptions, automated machines, other healthcare personnel interruptions, eg, nurse)
Sample Quality Related Event Form
Quality-Related Event Documentation
I. QRE Prescription Data Prescription No.: ________________________
Attach copy of: □prescription □ label □ photo copy of vial (mark all available)
II. QRE Data
QRE Type: (select all that apply)
A. Prescription processing error: B. A failure to identify and manage:
(1) Incorrect drug (1) Over/under-utilization
(2) Incorrect strength (2) Therapeutic duplication
(3) Incorrect dosage form (3) Drug-disease contraindication
(4) Incorrect patient (4) Drug-drug interactions
(5) Inaccurate or incorrect (5) Incorrect duration of treatment
packaging, labeling, or directions
(6) Other: __________________ (6) Incorrect dosage
(7) Drug-allergy interaction
(8) Clinical abuse/misuse
Prescription was received by the pharmacy via:□ telephone □ written □ computer □ fax
Prescription was: □ new □ refill
III. QRE Contributing Factors
Day of the week and time of QRE: ____________________________________________________________________
# of new prescriptions: _____________ # of refill prescriptions: ____________RPh to Tech ratio: ____________
RPh staff status: □ regular staff □ occasional/substitute staff
# of hours RPh on duty: ____________ Average # of prescriptions filled per hour: ____________
# of other RPh’s on duty: ____________ # of support staff on duty: ____________
Describe preliminary root contributors: ____________________________________________________________________________________________________________________________________________
Describe remedial action taken: ____________________________________________________________________________________________________________________________________________
Name and title of preparer of this report: _______________________________________________________________________________________________
Date Completed __________________________________________________________________________________________________________________________
Quality Related Events can be positive or negative. Positive QREs are those events that have a desirable outcome for the patient. A negative QRE is an event that is undesirable, untoward, or detrimental to the patient or healthcare process.
Below is a scenario of a positive QRE:
- REASON – Orlando pharmacist participating in the Florida patient care management program receives a prescription refill request for a cholesterol medication.
- ACTION – Pharmacist does an in-store cholesterol test and finds the patient not responding. Pharmacist calls the prescribing physician with results.
- RESOLUTION – Physician changes medication.
- OUTCOME – Patient responds to recommended change and cholesterol levels decrease.
Below is a scenario of a negative QRE:
- REASON – Hypertensive patient is in a hurry and presents a new prescription to the technician.
- ACTION – Pharmacy staff has difficulty reading the prescription but fills it based upon the pharmacist’s educated guess despite some drug utilization review alerts.
- RESOLUTION – Patient receives filled prescription in a timely and efficient manner.
- OUTCOME – Patient is admitted to hospital for uncontrolled blood pressure.
There are several contributing causes of negative quality related events. Common examples are below: (Click for video)
- Abbreviations
- D/C (Discharge – Discontinue)
- IU (International Unit – “IV” – “10”
- Q.D. (Once Daily – QID)
- SSRI (selective serotonin reuptake inhibitor or standardized service request item?)
- µg (microgram – milligram)
- Interruptions (telephone, verbal orders)
- Leading or trailing zeros
- Prescription volume
- Prescriber’s handwriting
- Product labeling and packaging
- Stress and fatigue
- Sound alike/look alike drug names
- Cisplatin – Carboplatin
- Zyrtec – Zyprexa
- Hydralazine – Hydroxyzine
Brain Check![GOK1]
Which may not lead to a negative QRE?
a. SSRI
b. milligram
c. IU
d. D/C
When managing a negative quality related event, be sure to listen to the patient or caregiver. Do not ignore their concern or dismiss it as a minor incident. Show genuine concern and assume that an error has occurred. Once the patient or caregiver has provided the information, research and investigate the QRE. It is good to show sympathy and apologize for the inconvenience while using professional judgment on accepting full responsibility. Compassionately sympathizing and saying “sorry” is not the same as accepting responsibility for the error and helps with positive communication. Be sure to document the QRE and notify the proper persons in charge.
There are several ways to prevent negative QREs:
1. During the dispensing process, avoid making an educated guess on prescriptions that may be questionable. Call the doctor’s office and verify the correct drug, the strength, directions, and other details. Do not assume.
2. Pay close attention to the patient’s profile. Verify it is the correct patient, the new prescription or refill is in sync with patient’s medical history, and always ask about allergies.
3. Do not ignore any warning signs or override computer messages without valid reason.
4.Pay close attention to changes in medications and appearances of dosage forms. Research further if insurance claims are denied as it may also serve as a warning.
5. Have policies and procedures in place and follow them. Compliance with policies can serve as checklist as it will promote consistency, an organized and conducive work environment, and minimal opportunities for errors.
CONTINUOUS QUALITY IMPROVEMENT
Continuous Quality Improvement (CQI) is defined as a management process that focuses on continually and systematically evaluating the organization’s work process. The goal is to improve the system process by making it more efficient and reducing the number of medication errors. The primary focus of CQI is to analyze system problems and not people. In community pharmacy, CQI can lead to enhanced patient safety through a reduction in medication errors and quality-related events.
According to Florida Administrative Code 64B16-27.300, the following are the requirements for creating a CQI that will help prevent quality related events.
- “Continuous Quality Improvement Program” means a system of standards and procedures to identify and evaluate quality-related events and improve patient care.
(2) “Quality-Related Event” means the inappropriate dispensing of a prescribed medication including:
(a) variation from the prescriber’s prescription order, including but not limited to:
- 1. Incorrect drug;
- 2. Incorrect drug strength;
- 3. Incorrect dosage form;
- 4. Incorrect patient; or
- 5. Inadequate or incorrect packaging, labeling, or directions
(b) a failure to identify and manage:
- 1. over-utilization or underutilization;
- 2. therapeutic duplication;
- 3. drug-disease contraindications;
- 4. drug-drug interactions;
- 5. incorrect drug dosage or duration of drug treatment;
- 6. drug-allergy interactions; or
- 7. clinical abuse/misuse
(3)(a) Each pharmacy shall establish a Continuous Quality Improvement Program which program shall be described in the pharmacy’s policy and procedure manual and, at a minimum shall contain; ————-
1. Provisions for a Continuous Quality Improvement Committee that may be comprised of staff members of the pharmacy, including pharmacists, pharmacy interns, pharmacy technicians, clerical staff, and other personnel deemed necessary by the prescription department manager of the consultant of record.
2. Provisions for the prescription department manager or the consultant pharmacist of record to ensure that the committee conducts a review of Quality Related Events at least every three months;
3. A planned process to record, measure, access and improve the quality of patient care;
4. The procedure for reviewing Quality Related Events
(b) As a component of its Continuous Quality Improvement Program, each pharmacy shall assure that following a Quality-Related Event, all reasonably necessary steps have been taken to remedy any problem for the patient.
(c) At a minimum, the review shall consider the effects on quality of the pharmacy system due to staffing levels, workflow, and technological support
(4) Each Quality-Related Event that occurs, or is alleged to have occurred, as the result of activities in a pharmacy, shall be documented in a written record or computer database created solely for that purpose. The Quality-Related Event shall be initially documented by the pharmacist to whom it is described and shall be recorded on the same day of its having been described to the pharmacist. Documentation of a Quality-Related Event shall include a description of the event that is sufficient to permit categorization and analysis of the event. Pharmacist shall maintain such records at least until the event has been considered by the committee and incorporated in the summary required in subsection (5) below.
(5) Records maintained as a component of a pharmacy Continuous Quality Improvement Program are confidential under the Health Insurance Portability and Accountability Act and are exempt from discovery pursuant to provisions of section 766.101, F.S. In order to determine compliance, the Department may review the policy and procedures and a Summarization of Quality related events. The summarization document shall analyze remedial measures undertaken following a Quality Related Event. The summarization document shall analyze remedial measures undertaken following a Quality-Related Event. No patient name or employee name shall be included in this summarization. The summarization shall be maintained for four years. Records are considered peer-review documents and are not subject to discovery in civil litigation or administrative actions.
There are several different CQI models, however they all focus on improving the quality of products, services, and processes. A specific model is the PDSA model, developed by Walter Shewhart, a pioneer in the field of quality control. The PDSA model is a four-step process which stands for Plan, Do, Study/Check, and Act.
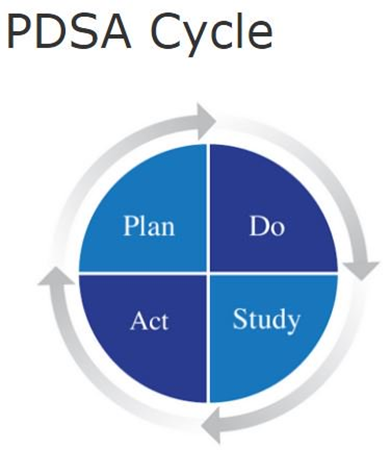
Guide to Continuous Quality Improvement | Smartsheet
There are several variations of the PDSA model, including the FOCUS-PDCA, a nine-step model: Find, Organize, Clarify, Understand, Select, Plan, Do, Check, and Act. The Six Sigma model is another popular model used in business management. Pharmacists have many options to choose from, all of which can help minimize medication errors, potentially promote a safer work environment.
The purpose of CQI program is to ensure that the entire pharmacy team are proactively involved in ways to be more efficient and effective. The team may be comprised of all staff members of the pharmacy, including pharmacists, registered pharmacy interns, registered pharmacy technicians, clerical staff, and other personnel deemed necessary by the pharmacist in charge or the consultant pharmacist of record. There are times when employees may not adjust well to change but the focus is on improvement. A successful CQI program must be ongoing and consistent. A CQI program shall require that the pharmacist in charge or the consultant pharmacist of record ensure that a review of quality-related events occurs at least quarterly, contain a planned process to record and assess QREs, include a process for documenting actions to improve the quality of patient care, and maintain a summary of the documented actions. The review should consider environment and systems-based contributing factors. The pharmacist in charge is the leader who can articulate a clear understanding of the program purpose and intent. Policy and procedure documents should be available to ensure compliance, to document quality related events, and to provide evidence of pharmacy staff trainings and meetings. The policy and procedures document manual should include the following:
- Purpose of the program
- Definitions used in the program
- Policy on how to report a Quality-related event
- Policy for program meetings including frequency of meetings, staff to attend, and involvement of either the Pharmacist-in-Charge or Consultant Pharmacist.
- Policy for maintaining program integrity and confidentiality of information
- Policy for staff education and ongoing training of employees related to CQI program
- Policy for employee engagement and communication of changes made related to the program
- Policy for maintaining summarization documents for board inspection.
All reported QREs should be documented. They should be documented by the individual who discovers the event or the individual to whom it is initially reported. Documentation of QREs should include a description of the event. Pharmacies should maintain these records at least until the event has been presented and incorporated in a summary of documented actions. Presentations at the meetings allow open dialogue of any necessary changes that may be required to avoid future errors or minimize patient harm. CQI programs can be confidential. Efforts should be made to remove any patient names or employee names in summary reports. These reports should be maintained for 4 years and be made available within 3 business days of a request by the board’s inspectors. Continuous quality improvement records are considered peer-review documents and not subject to any civil litigation or administrative actions. Ongoing improvement is imperative to avoid reducing medication errors.
GROUNDS FOR CITATION
The Florida Board of Pharmacy has disciplinary guidelines in place for violations. According to Rule 64B16-30.003, there is a $250 fine and completion of an approved CE course in the prevention of medication errors of no less than 8 hours for ‘Use in the compounding of a prescription, or furnishing upon prescription, an ingredient or article different in any manner from the ingredient or article prescribed, except as authorized in section 465.019(6) or 465.025, F.S.; or dispensing a medication with dosage instructions different in any way than prescribed, provided that the medication was not used or ingested’. (Section 465.016(1)(g), F.S.). Once the citation becomes a final order, the citation and complaint become a public record pursuant to chapter 119, F.S., unless otherwise exempt from the provisions thereof. The citation and complaint may be considered as aggravating circumstances in future disciplinary actions pursuant to paragraph 64B16-30.001(3)(a), F.A.C.
Brain Check!
QRE reports should be available within __ business day(s) of a request by the board inspection.
a. 1
b. 3
c. 5
d. 7
CONCLUSION
Medication errors have affected many patients and is a major cost burden. Public safety is the number one priority of all healthcare personnel. Medication errors may be prevented by having and following processes, systems, proper training, and carefully implemented technology in place. Continuous quality improvement meetings allow pharmacy staff to discuss necessary steps required to prevent or minimize quality related events.
Pharmacy staff can play a key role in mitigating medication errors by following the 5 Rights: dispensing the right dose of the right medication to the right patient at the right time and by the right route. CQI programs are beneficial and ensure that the entire pharmacy team are proactively involved in ways to be more efficient and effective in reducing medication errors.
SAMPLE CQI FORM
CONTINUOUS QUALITY IMPROVEMENT MEETING REPORT FORM
Date of Report:________________________________________ Date of Meeting:_________________________________________ Quarter:_______________________________________
Name of Pharmacy Employees in Attendance:
_____________________________________________________________________________________________________________________________________________________________________________
Overview of Quality Related Events (QREs)
Identify Incident Type:
_____________________________________________________________________________________________________________________________________________________________________________
Action plan to be taken to prevent recurrence of each incident reviewed:
_____________________________________________________________________________________________________________________________________________________________________________
Identify Incident Type:
_____________________________________________________________________________________________________________________________________________________________________________
Action plan to be taken to prevent recurrence of each incident reviewed:
_____________________________________________________________________________________________________________________________________________________________________________
(Use multiple pages as needed to cover all incidents in current quarter)
Contributing Factors:
Staffing Levels
Workflow
Technological Support
Other
Person responsible to inform associates not present of results and action plans:__________________________________________________________________________
Date Completed: ________________________________________________________________________________________________________________________________________________
REFERENCES
Medication Error Statistics 2023 | SingleCare; Medication Erros: Common Types, Causes, and Prevention – Medcom, Inc. (medcominc.com). Accessed January 2023
Medication | definition of medication by Medical dictionary (thefreedictionary.com)
Medication errors: what they are, how they happen, and how to avoid them | QJM: An International Journal of Medicine | Oxford Academic (oup.com) Forms of Drugs| Different Types of Dosage forms with examples. (studyread.com) Updated August 23, 2022
Root Cause Analysis with Examples – Bing video. Accessed January 2023
Special Edition: Tall Man Lettering; ISMP Updates Its List of Drug Names with Tall Man Letters | Institute For Safe Medication Practices Home | Institute For Safe Medication Practices (ismp.org); Look-Alike Drug Names with Recommended Tall Man (Mixed Case) Letters | Institute For Safe Medication
Practices (ismp.org). Accessed January 2023
Patient Engagement and Education | Agency for Healthcare Research and Quality (ahrq.gov). Accessed February 2023
The 5 Rights of Medication Administration – Pharmacology | Lecturio – 5 Rights of medication administration. Accessed February 2023
What are Quality Related Events? – Safe Assured – Dalhousie University. Accessed February 2023.
64B16-30.003: Citations – Florida Administrative Rules, Law, Code, Register – FAC, FAR, eRulemaking (flrules.org). Accessed February 2023


No Comments